Human immunodeficiency virus continues to pose a threat to global human health. Despite promising therapies such as antiretroviral (cART) and even bone marrow replacement therapies, HIV-1 replication is controlled, but no current therapy affects the latent viral genome inserted in human cells. The HIV virus is currently treated by antiretroviral therapy that affects viral replication by controlling the disease. Although these drugs are very effective, you should consider their cost and the fact that people should take them chronically. Therefore, many people would prefer to completely eliminate the virus from their genome, even those latent viral reservoirs [1]. The delta 32 mutation causes an alteration in the CCR5 chemokine receptor. Said receptor serves for the admission of the acquired immunodeficiency virus (HIV) to the CD4 T cells of the immune system. People with this rare mutation do not become infected with HIV-1 and do not develop AIDS syndrome [2].
Many attempts have tried to expand this condition in people who are infected with HIV-1 as a gene therapy. The goal is to reduce the viral load to less than 200 copies per milliliter of blood, which allows the immune system to take over the virus and people are not able to transmit the virus sexually. In 2014, Thebes and collaborators injected 12 patients with CD4 T cells expressing a protein called “Zinc Finger” or “zinc finger” fused to a DNA nuclease. This “trojan horse” is known as ZFN (“zinc finger nuclease”) consists of injecting 10 billion recombinant CD4 T cells, intravenously in HIV-positive patients, to whom antiretroviral therapy was discontinued. Zinc fingers or ZFN recognize a region in the viral genome and eliminate it. Using ZFN, there was a serious adverse effect from transfusion and only 25% of patients had undetectable HIV-1 status [3].
In 2019, a bone marrow transplant was performed for a patient with both acute lymphoblastic leukemia and HIV positive. Hematopoietic stem cells and progenitors (HSPC) were edited with CRISPR deficient in CCR5. No adverse effects of HSPC cells (CCR5-/CRISPR) were observed and long-term establishment was achieved. However, only 5 % of lymphocytes have an interruption of CCR5 [4].
Cases have been reported where conventional bone marrow treatment using a donor with the CCR5 mutation can prevent HIV infection. In such cases, people have been declared HIV-free by such therapy. Recently, at the CROI 2022 congress, new and successful treatment was reported in a woman with HIV remission. The HIV-positive person had developed acute myeloid leukemia. Both diseases were treated with antiretrovirals and chemotherapy, respectively. In 2017, he underwent a treatment where umbilical cord stem cells from a donor with the CCR5 mutation and stem cells from a bone marrow donor. After 37 months of dual transplantation, antiretroviral therapy was discontinued and HIV is currently undetectable in the serum [5].
Bone marrow transplantation is a costly, high-risk procedure and very few medulla donors are available with the delta 32 mutation. Additionally, they require months of donor search and patient preparation and laboratory methods on a case-by-case basis. Could a comprehensive therapy strategy be implemented to eliminate HIV in patients on antiretroviral therapy at a reasonable cost?
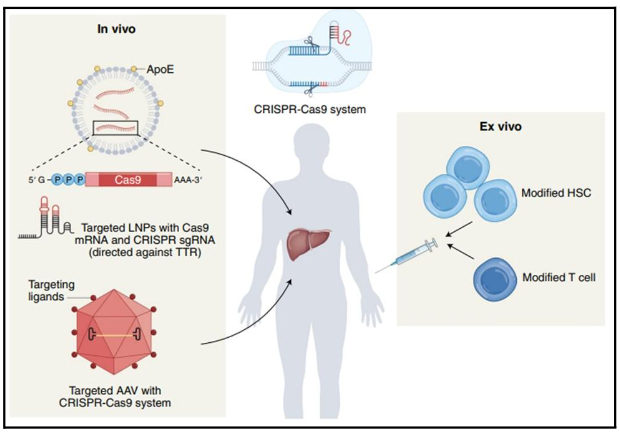
The main limitation of therapies is that the virus can remain inactive for years without producing copies, but with an infectious viral load. The HIV reservoirs in the genome are unattainable to the immune system and current therapies. The CRISPR genetic editing system promises to inactivate the HIV-1 viral genome in patients with active antiretroviral therapy. This is due to recent advances with in vivo CRISPR therapy. There are successful examples of “ex vivo” therapy where cells from the patient’s body are genetically modified and then reintroduced. This procedure is costly and specialized, and not all conditions qualify for “ex vivo” cell isolation therapy.
The next challenge for the CRISPR system is the “in vivo” delivery of the genetic editing machinery. In this configuration genetic editing occurs within the patient’s body. Transthyretin amyloidosis (ATTR) is characterized by the accumulation of this poorly folded protein in tissues of the nervous system and heart. The CRISPR NTLA-2001 system consists of lipid nanoparticle (LNP) that are transported to the liver by blood transfusion. Hepatocytes absorb LNP/CRISPR, and begin the process of genetic editing. Similarly, it proceeds in the treatment of hereditary angioedema disease, where the KLKB1 gene that encodes for the protein kallikrein must be inactivated. The overaccumulation of this protein produces bradykinin causing severe inflammations that can become fatal. Intellia Therapeutics employs this strategy because the liver easily absorbs fat micelles (LNP) and it is relatively simple to treat conditions related to the proper functioning of this organ. Using this method, they report that the CRISPR system reduced harmful protein (transthyretin); in more than 90 percent of a total of 12 people with amyloidosis, a genetic disease that can cause heart failure [6].
Currently, the challenge for CRISPR therapy is to bring genetic editing to other organs. LNP technology does not deliver CRISPR to organs such as the brain and lungs. Researchers are now wondering if a pathogen such as HIV-1 could be stopped and attacked the genome inserted into CD4 T-cell reservoirs. The inserted virus needs its entire genome to replicate. Therefore, if CRISPR removes fragments from it, it would inactivate the virus’s ability to infect.
The first studies have been promising. Laboratory mice modified with HIV-1 genome insertions showed that CRISPR can remove regions of the virus inserted into the host’s DNA. The same group of researchers demonstrated a similar effect in apes, when using the tool to edit the genome of the SIV virus (“simian immunodeficiency virus”). The removal of the Gag gene in viral DNA in both animal models makes the virus undetectable in the blood. This research published in the journal Nature in 2019 shows that the virus can be permanently eliminated from the animal host [7, 8].
The previous history motivated scientists to implement trials on HIV-positive patients with active antiretroviral therapy. Excision Biotherapeutics conducts the study in nine patients in which CRISPR is delivered in vivo using modified adenovirus to introduce editing machinery to CD4 T cells (Figure 2). Such a system should have the following characteristics: A) Do not cause side effects in people. B) The system should not edit regions out of context, i.e., alter the human genome and only edit the HIV genome. C) A viral region is chosen that is conserved so as to ensure little variation in viral DNA and a high rate of editing. D) Edited CD4 T cells must remain in that condition over time. Genetic editing must be stable. E) It is desirable that the CRISPR system behaves in the same way in all people.
People in the study will receive the transfusion of gene therapy and stop using antiretrovirals. The viral load and CD4 T cell count will be measured before and after transfusion. The main objective is to edit the largest number of lymphocytes to obtain less than 200 copies of viruses per milliliter, which makes the patient seronegative and unable to transmit the virus sexually [9].
There is still very little information about the use of this therapy in humans. Moreover, CRISPR system behavior information in an in vivo format is a developing technology. If the latent proviral genome is inactivated in enough T CD4, the immune system should be able to control the viral infection without developing AIDS. At least that is the current hypothesis of scientists. There are many questions about the functioning of the system and there are still few alternatives for delivering the gene editing system to white cells in vivo. However, the CRISPR system has already shown promising results for genetic diseases. Will similar results be achieved to control a viral infection such as HIV-1?
References
1.- Zhihao Zhang, Wei Hou, Shuliang Chen, Updates on CRISPR-based gene editing in HIV-1/AIDS therapy. 2022. Virologica Sinica, Volume 37, Issue 1, 2022, Pages 1-10, ISSN 1995-820X, https://doi.org/10.1016/j.virs.2022.01.017.
2.- Samson, M., Libert, F., Doranz, B. et al. Resistance to HIV-1 infection in Caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature 382, 722–725 (1996). https://doi.org/10.1038/382722a0
3.- Tebas, Pablo and Stein, David and Tang, Winson W. and Frank, Ian and Wang, Shelley Q. and Lee, Gary and Spratt, S. Kaye and Surosky, Richard T. and Giedlin, Martin A. and Nichol, Geoff and Holmes, Michael C. and Gregory, Philip D. and Ando, Dale G. and Kalos, Michael and Collman, Ronald G. and Binder-Scholl, Gwendolyn and Plesa, Gabriela and Hwang, Wei-Ting and Levine, Bruce L. and June, Carl H. Gene Editing of CCR5 in Autologous CD4 T Cells of Persons Infected with HIV. 2014. New England Journal of Medicine, vol 370, number 10, pp 901-910. https://doi.org/10.1056/NEJMoa1300662
4.- Xu Lei, Wang Jun, Liu Yulin, Xie Liangfu, Su Bin, Mou Danlei, Wang Longteng, Liu Tingting, Wang Xiaobao, Zhang Bin, Zhao Long, Hu Liangding, Ning Hongmei, Zhang Yufeng, Deng Kai, Liu Lifeng, Lu Xiaofan, Zhang Tong, Xu Jun, Li Cheng, Wu Hao, Deng Hongkui, Chen Hu. 2019. CRISPR-Edited Stem Cells in a Patient with HIV and Acute Lymphocytic Leukemia. New England Journal of Medicine; vol 381, number 13, pp 1240-1247. https://doi.org/10.1056/NEJMoa1817426
5.- First case of HIV cure in a woman after stem cell transplantation reported at CROI-2022. World Health Organization Report 2022. https://www.who.int/news/item/24-03-2022-first-case-of-hiv-cure-in-a-woman-after-stem-cell-transplantation-reported-at-croi-2022
6.- Gillmore Julian D., Gane Ed, Taubel Jorg, Kao Justin, Fontana Marianna, Maitland Michael L., Seitzer Jessica, O’Connell Daniel, Walsh Kathryn R., Wood Kristy, Phillips Jonathan, Xu Yuanxin, Amaral Adam, Boyd Adam P., Cehelsky Jeffrey E., McKee Mark D., Schiermeier Andrew, Harari Olivier, Murphy Andrew, Kyratsous Christos A., Zambrowicz Brian, Soltys Randy, Gutstein David E., Leonard John, Sepp-Lorenzino Laura, Lebwohl, David. 2021. New England Journal of Medicine, vol 385, No. 6, pp 493-502. https://doi.org/10.1056/NEJMoa2107454
7.- Dash, P.K., Kaminski, R., Bella, R. et al. Sequential LASER ART and CRISPR Treatments Eliminate HIV-1 in a Subset of Infected Humanized Mice. Nat Commun 10, 2753 (2019). https://doi.org/10.1038/s41467-019-10366-y
8.- Mancuso, P., Chen, C., Kaminski, R. et al. CRISPR based editing of SIV proviral DNA in ART treated non-human primates. Nat Commun 11, 6065 (2020). https://doi.org/10.1038/s41467-020-19821-7
9.- Büning, H., Schambach, A. A first step toward in vivo gene editing in patients. Nat Med 27, 1515–1517 (2021). https://doi.org/10.1038/s41591-021-01476-6