Head of Tissue Engineering and Synthetic Biology
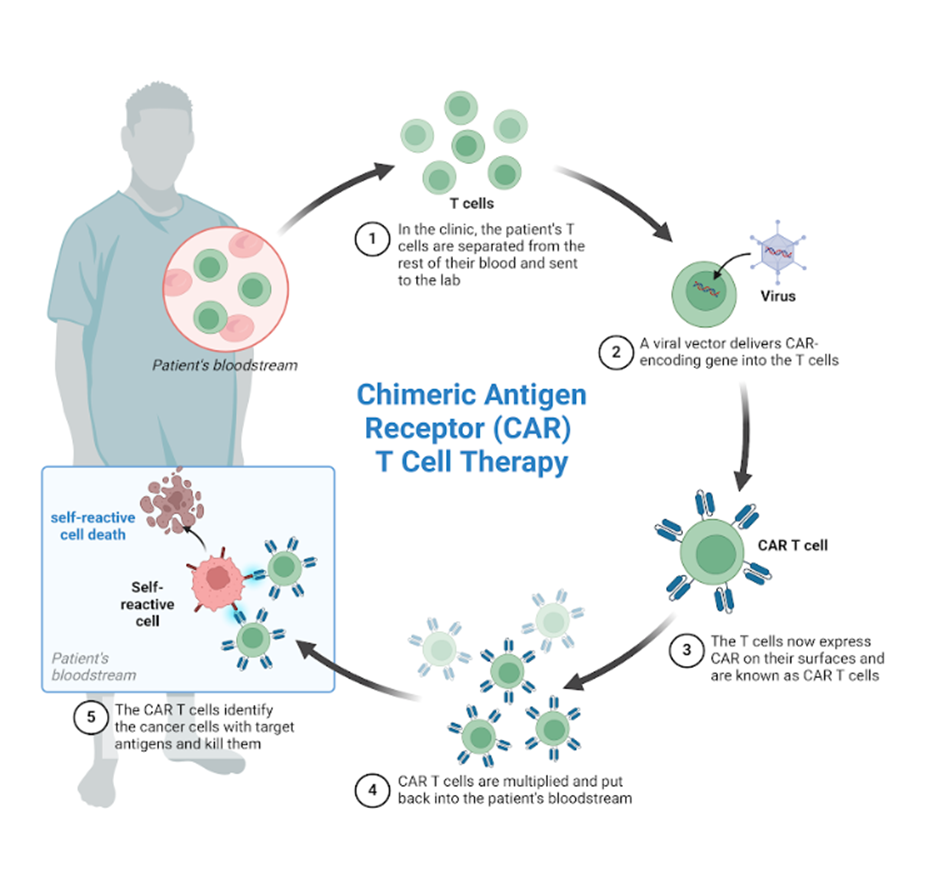
Chimeric Antigen Receptors (CAR) are receptors designed using genetic engineering techniques, with the aim of targeting primarily T lymphocytes and NK cells, to recognize and eliminate cells that express a specific antigen. Binding of CAR to target antigens present on the cell surface occurs independently of the major histocompatibility complex (MHC), resulting in robust activation of T cells and powerful immune responses.
The four main components of CAR receivers are:
1. Extracellular target antigen binding domain: Gives it the specificity to bind to a given antigen. To recognize a given antigen and induce T-cell activation, the CAR antigen binding affinity must be high enough, but not high enough to cause death induced by activation of CAR-expressing T cells and trigger toxicities.
2. Region hinge: Provides flexibility and contributes to length to allow the antigen binding domain to access the target epitope.
3. Transmembrane domain: Its most studied function is to anchor the CAR receptor to the T cell membrane.
4. One or more intracellular signaling domains: The most common co-stimulant domains are CD28 and 4-1BB (CD137); which are associated with high patient response rates.
The manufacturing process of CAR-T cells begins with the isolation of peripheral mononuclear cells (PBMCs), by the Ficoll-Paque density gradient technique or by leukoperesis, then CD4+ and/or CD8+ T cells depending on the desired product, enrichment and activation is performed by exposing cells to pearl-bound anti-CD3 and anti-CD28 activating antibodies. Then perform genetic modifications of isolated T cells, using viral or non-viral approaches
Autoimmune disorders are a broad category of diseases with complex pathology. These disorders develop when the body’s own immune cells (particularly self-reactive B and T cells) initiate abnormal attacks on their own tissues through a variety of effector pathways. These diseases arise from a combination of genetic predisposition and environmental factors.
To prevent self-reactive immune cells from attacking host organs, the treatment of autoimmune diseases is still to use broad immunosuppressive drugs, usually not directed, such as glucocorticoids and nonsteroidal anti-inflammatory drugs, or their blocking with specific antibodies, such as antibodies against CD20 (Rituximab) or BAFF (Belimumab), antibodies against T cells, such as anti CD52 (alemtuzumab), or against proinflammatory cytokines as antibodies against TNF-a (infliximab). Autoimmune diseases can also be treated by hematopoietic cell transplantation which may be autologous or allogeneic.
Cell therapy for autoimmune diseases has two approaches; eliminate and cushion the self-reactive immune cells.
In a recent article published in The New England Journal of Medicine, researchers treated 8 patients with systemic lupus erythematosus, 3 patients with idiopathic inflammatory myositis and 4 patients with systemic sclerosis; with a single dose of chimeric receptor (CAR) CD19 T-cell infusion. All patients in the study had negative responses to at least two previous immunosuppressive treatments.
Nine days after infusion, CAR T cells reached peak concentrations (146 per microliter), with B cells CD19+ removed from the peripheral blood after 7 days.
After six months, patients with systemic lupus erythematosus presented with remission, the disease being absent for 29 months. In the three patients with idiopathic inflammatory myositis after three months creatinin kinase levels and muscle function normalized, all patients discontinued corticosteroids and other immunosuppressive drugs.
The data shown in this study provide new evidence of the safety and short-term efficacy of CAR-T cell CD19 therapy for autoimmune diseases, but clinical studies are necessary.
Bibliografia
- Blache, U. et al. (2023) ‘CAR T cells for treating autoimmune diseases’, RMD Open, 9(4), pp. 1–9. Available at: https://doi.org/10.1136/rmdopen-2022-002907.
- De Marco, R.C., Monzo, H.J. and Ojala, P.M. (2023) ‘CAR T Cell Therapy: A Versatile Living Drug’, International Journal of Molecular Sciences, 24(7), pp. 1–22. Available at: https://doi.org/10.3390/ijms24076300.
- Müller, F. et al. (2024) ‘CD19 CAR T-Cell Therapy in Autoimmune Disease — A Case Series with Follow-up’, The New England Journal of Medicine, 390(8), pp. 687–700. Available at: https://doi.org/10.1056/NEJMoa2308917.