Laboratory Technician
Bioengineering and Chemical Engineering Department
Fireworks are used in many events around the world, are synonymous with celebration and a fascinating example of chemistry and engineering in action. Over the years the technology around them has been changing, but how do they work? How do they form different patterns and colors? What is the science behind such an amazing explosion of lights, colors and varied shapes? Let’s see some answers to these questions…
Pyrotechnic devices contain chemicals; generally, it is composed of an oxidizer, a fuel, a binder and optionally a chemical that improves color. The lights, colors and sounds of fireworks come from these chemicals.
Gunpowder is the fuel most used in fireworks which provides the energy to make the cartridge fly in the air; oxidizers can be: nitrates, chlorates and perchlorates; whose function is to generate enough oxygen for combustion; the binder (dextrin, rubber, resin, etc.) holds all other components together and finally metal salts produce specific colors.

LIGHTS, SOUND AND SHAPES
Colors can be formed by two mechanisms: luminescence and incandescence.
In incandescence occurs by heating, heat causes the material to begin to glow and emits light at different wavelengths (infrared light, then red, orange, yellow and white as the substance gets hotter and hotter). When you control the temperature of an artificial fire, the brightness of the metal compounds can be manipulated to have the desired color at the right time. The creation of blue and green lights requires a higher temperature, which is impractical in fireworks; therefore these colors are achieved by the luminescence mechanism. In luminescence no heat is required for a body to emit light and so it occurs at lower temperatures. Light is generated by the electrons of the metal atom, which when absorbing energy from the heat generated in the explosion jump to a higher level, becoming excited electrons and then return to its lower energy ground state, releasing a photon of light of a certain energy and characteristic color.
The explosion of an artificial fire occurs in two steps: the aerial projectile is fired into the air and then explodes in the air, many meters from the ground. During the explosion of the projectile not only gases are produced quickly, but they also heat and expand following Charles’s law (If the pressure is constant, as the temperature of an enclosed gas increases, the volume also increases). The rumble is due to the expansion of gases at a faster rate than the speed of sound.
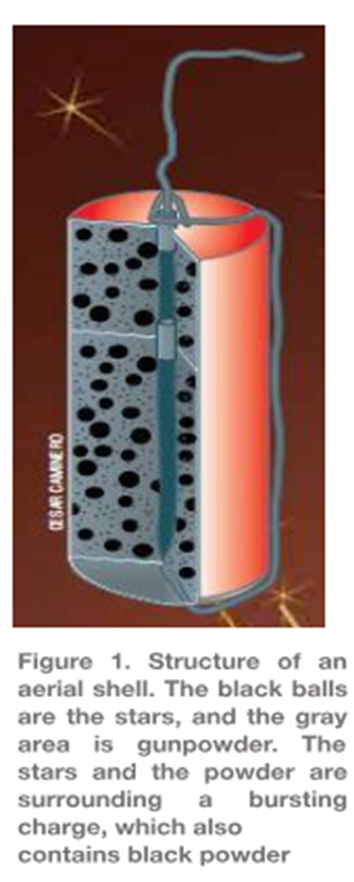
Along the same line, the arrangement of the stars (see figure 1) determines the shape of the lights; if they are randomly arranged on the projectile, they will spread evenly in the sky after the explosion. But, if carefully grouped into certain patterns, the fireworks will have a specific shape because the stars are sent in point directions during the explosion.
That is why, in terms of sound and forms of fireworks, everything boils down to construction of an air projectile!
ENVIRONMENTAL IMPACT AND FUTURE
While fireworks are a spectacle for our eyes, it should also be considered that the remains of the projectile generate waste, in addition, combustion generates smoke and particles that pollute the air. A recent study designed flares providing a functional coating that has potential properties to inhibit moisture absorption and control gaseous particle emissions. Therefore, it is necessary to continue with research and search for more eco-friendly alternatives, such as biodegradable projectiles or forms of green combustion.
Today, computers play a key role in both the development of fireworks and the design of their displays. Computer programs can be used to synchronize the shooting of thousands of fireworks from a single control panel.
Designing a fireworks show is both an art and a science. Fireworks designers must understand the chemical properties of various compounds to produce the desired colors. They must consider the physics of the dynamics of explosions to create the forms and patterns required. In addition, they must also have an artistic vision to choreograph these elements in a way that offers a captivating spectacle.
References
- Utkarsha W., Girivyankatesh H, Suraj J., Anirban M., Rakesh K., Sadhana R.; “Glazing of the fireworks: Functional coating materials for enhancing the shelf life and reducing emissions”; Environmental Technology & Innovation 28 (2022) 102926; https://doi.org/10.1016/j.eti.2022.102926
- N. Selvakumar, A. Azhagurajan, A. Suresh; “Experimental analysis on nano scale flash powder composition in fireworks manufacturing”; J Therm Anal Calorim (2013) 113:615–621; DOI 10.1007/s10973-012-2749-9
- 10 Chemmatters, October 2010.