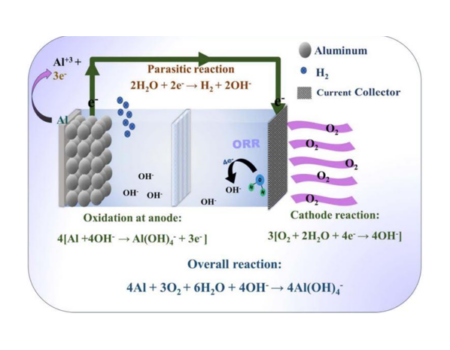
Senior Laboratory Responsable
Bioiengineering and Chemical
Engineering Department
Down syndrome is a genetic condition caused by nondisjunction in meiosis, which results in the presence of an extra chromosome in pair 21. This syndrome is characterized by a typical physical appearance, intellectual disability and developmental delays, and can also be associated with heart or thyroid gland diseases. For decades, research has been conducted on how to improve the quality of life of people with this condition, but no attempt had ever been made to eliminate the cause of the problem: the extra chromosome. However, a recent study published in PNAS Nexus has shown that it is possible to do so with a gene editing technique called CRISPR-Cas9.
What is Crispr-Cas9?
CRISPR-Cas9 is a revolutionary tool that allows precise DNA editing. It works like a “molecular scissors” that can cut specific sections of DNA, allowing genetic fragments to be removed, added or modified. In this case, researchers used CRISPR-Cas9 to remove the extra chromosome in cells with Down syndrome.
CRISPR-Cas9 has been used in various research to correct genetic mutations in inherited diseases and birth defects. This technology has revolutionized biotechnology and medicine, allowing precise manipulation of the genome with applications ranging from basic research to the creation of personalized treatments.
How did they do it?
Hashizume and his team designed a version of CRISPR-Cas9 to only attack the extra chromosome present in Down syndrome cells, leaving the other two normal chromosomes 21 unaffected. To achieve this, they used a technique called “allele-specific chromosome cutting” (AS-Cas9).
First, they identified unique DNA sequences on the extra chromosome. Then, they programmed CRISPR-Cas9 to cut only in those regions. By making multiple cuts in the extra chromosome, the cells were unable to repair it properly and ended up deleting it.
The research was conducted on induced pluripotent stem (iPS) cells, which have the ability to become any type of cell in the human body that are reprogrammed to regain a state similar to that of embryonic stem cells. These cells are obtained from adult cells, such as skin fibroblasts. Thanks to this ability, iPS cells can differentiate into any tissue, making them a powerful tool for regenerative medicine and genetic disease research. In this work, fibroblasts from a person with Down syndrome (skin cells) were tested to evaluate whether the elimination of the extra chromosome could occur in already differentiated cells.
Karyotype analysis before and after treatment
To evaluate the effects of the elimination of the extra chromosome, an analysis of the karyotype in metaphase was performed by microscopy before and after treating IPS cells with the M2-As × 13 vector.
• Before treatment: Images were captured in G bands metaphase and the IPS cell line kariogram was performed with original trisomy 21. In this analysis, all cells showed karyotypes of 47, xy,+21 in 19 (100%) of the 19 metaphases observed.
• After treatment: snapshots representative of metaphase were taken after the application of the vector M2-As × 13 in the original Trisomy 21 cells. In this case, the analysis revealed that 62.5% of the cells (25 of 40 metafase) continued to present the 47, xy,+21 affectionate, while 37.5% of the cells (15 of 40 metafasses) had corrected their chromosomal number to 46, xy.
The G band analysis did not detect any apparent structural variant or additional numerical chromosomal abnormalities, which suggests that the elimination of the extra chromosome occurred specifically and without causing collateral damage in the genome (see Figure 1).
Results and what they mean?
The study showed that, after the elimination of the extra chromosome, the cells behaved again similar to normal cells. Genes expression was restored and some cellular functions improved, including cell growth and oxidative stress reduction, a common problem in cells with Down syndrome.
This is important because many of the clinical manifestations of Down Syndrome, such as cognitive and cardiac problems, are related to overexpression of genes on extra chromosome 21. If this additional chromosome can be eliminated in early stages of development, there could be a significant improvement in cell function and, potentially, in the symptoms of the disease.
What does this mean for the future?
This discovery opens the door to possible genetic therapies to treat Down syndrome. Although the investigation was done in cells cultivated in the laboratory, is a first crucial step towards the possibility of developing treatments for people with this condition.
However, there are still important challenges. First, you should investigate whether this technique is safe in complete organisms and not only in isolated cells. Second, there are ethical issues about the modification of human DNA that must be discussed before applying this technology in people.
It is also essential to determine if the elimination of chromosome in a developing embryo could prevent the appearance of Down syndrome without causing other adverse effects. While the results in the laboratory are encouraging, bringing this research to the clinic will require many more tests and strict regulations.
Conclusion
The use of CRISPR-CAS9 to eliminate extra chromosome in cells with Down syndrome is a promising advance. Although there is still much to investigate, this study represents a more step towards the development of genetic therapies that could change the lives of many people in the future. Science continues to advance and with it, the hope of improving the health and well -being of humanity.
While it is not clear when this technology could be applied in humans, what is evident is that we are in an era where genetic edition could transform our understanding and treatment of many genetic diseases.
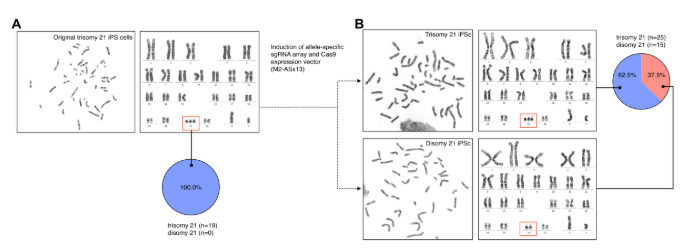
Figure 1: Análisis de Cariotipo
Reference Hashizume, R. (2025). Trisomic rescue via allele-specific multiple chromosom cleavage using CRISPR-Cas9 in trisomy 21 cells. PNAS Nexus, 4(2), 1–13. https://doi.org/10.1093/pnasnexus/pgaf022